
Patient case studies
Publishing date: 5th December 2019
These interactive patient case studies allow you to learn about real world data and the use of GLP-1RAs in clinical decision making. These fictional cases, written by leading international experts, feature a patient profile and the latest published data, set against points for clinical decision making for you to learn and understand the rationale behind the multiple choice answers.
CME credits are no longer available
GLP-1RAs in patients with renal impairment
This expert authored self-assessment looks at how to manage a patient with diabetes and renal impairment, including the most appropriate treatment to reduce the risk of deteriorating renal function.


Weight Management
This expert authored self-assessment looks at how to manage a severely overweight patient with diabetes, whose aim is to have bariatric surgery.
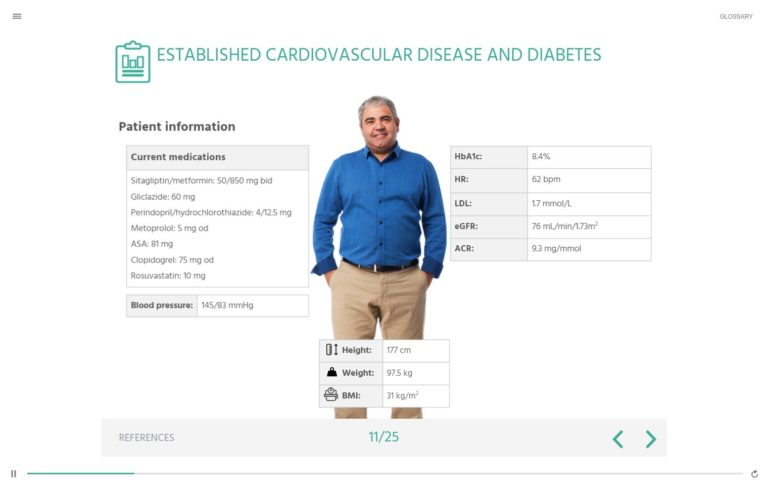
Established Cardiovascular Disease and Diabetes
Test your knowledge about the use of GLP-1RAs in diabetes treatment with this CME- accredited interactive patient case study. This expert authored self-assessment looks at how to manage a patient with diabetes and established cardiovascular disease, including the most appropriate treatment to lower the risk of cardiovascular complications.
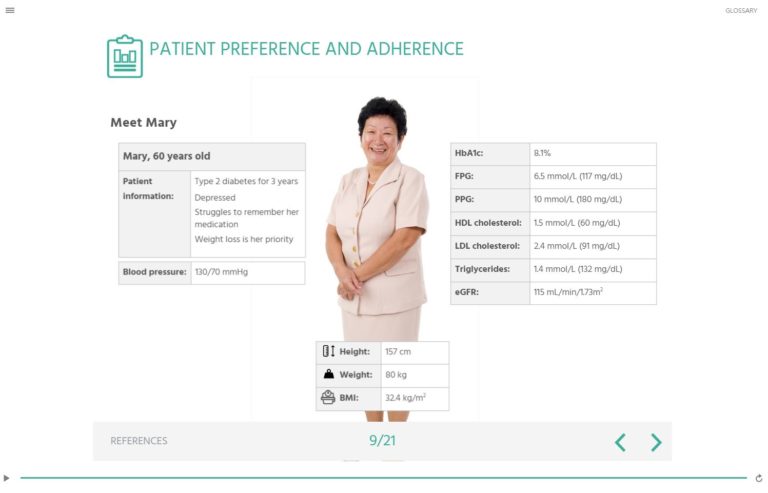
Patient Preference and Adherence
Test your knowledge about the use of GLP-1RAs in diabetes treatment with this CME- accredited interactive patient case study. This expert authored self-assessment walks you through a case of suboptimal medication adherence and discusses the need for patient preferences in creating management plans.
Please register and/or login to access these case studies
Create a free account
Login
Professor Melanie Davies
 Melanie Davies is a clinician with over 25 years’ experience working as a diabetologist and physician and since 2006 is Professor of Diabetes Medicine at the University of Leicester, in the UK. She is an National Institute of Health Research (NIHR) Senior Investigator, one of only a handful in diabetes in the UK, Director of the NIHR Leicester-Loughborough Diet, Lifestyle and Physical Activity Biomedical Research Unit, Director of a Clinical Trial Unit, Lead for Division 2 of the Clinical Research Network East Midlands and Principal Investigator on a number of large global studies in the field of diabetes, obesity and cardiovascular disease. Professor Davies is the global PI on the SCALE Obesity trial, a large multinational study investigating management of obesity with GLP-1 analogue.
Melanie Davies is a clinician with over 25 years’ experience working as a diabetologist and physician and since 2006 is Professor of Diabetes Medicine at the University of Leicester, in the UK. She is an National Institute of Health Research (NIHR) Senior Investigator, one of only a handful in diabetes in the UK, Director of the NIHR Leicester-Loughborough Diet, Lifestyle and Physical Activity Biomedical Research Unit, Director of a Clinical Trial Unit, Lead for Division 2 of the Clinical Research Network East Midlands and Principal Investigator on a number of large global studies in the field of diabetes, obesity and cardiovascular disease. Professor Davies is the global PI on the SCALE Obesity trial, a large multinational study investigating management of obesity with GLP-1 analogue.
In the last three years she has had more than 180 peer review publications and has been awarded, in the last five years, over £30,000,000 of external reviewed grant funding.
Disclosures
- Consultant, advisory board member and speaker fees: Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca and Janssen, an advisory board member for Servier and as a speaker for Mitsubishi Tanabe Pharma Corporation and Takeda Pharmaceuticals International Inc.
- Grants in support of investigator and investigator initiated trials from Novo Nordisk, Sanofi-Aventis, Lilly, Boehringer Ingelheim and Janssen.
Dr Stewart Harris
 Stewart Harris is a Professor at the Schulich School of Medicine & Dentistry, Western University, London, Ontario, Canada; he holds the Canadian Diabetes Association Chair in Diabetes Management and the Ian McWhinney Chair of Family Medicine Studies. Dr Harris is also Medical Director at the Primary Care Diabetes Support Program at St. Joseph’s Health Care – an integrated team-based diabetes clinic for disenfranchised and marginalized populations.
Stewart Harris is a Professor at the Schulich School of Medicine & Dentistry, Western University, London, Ontario, Canada; he holds the Canadian Diabetes Association Chair in Diabetes Management and the Ian McWhinney Chair of Family Medicine Studies. Dr Harris is also Medical Director at the Primary Care Diabetes Support Program at St. Joseph’s Health Care – an integrated team-based diabetes clinic for disenfranchised and marginalized populations.
He has published over 250 articles in peer reviewed journals and has participated extensively in clinical practice guideline development.
He is the recipient of the Ontario Ministry of Heath Career Scientist award, the Dr Gerald S. Wong Service Award of the Canadian Diabetes Association, the Hellmuth Prize for Achievement in Research at Western University, and is a Fellow in the Canadian Academy of Health Sciences. On July 1st 2015, Dr Harris was appointed to the Order of Canada for his contributions to the development of strategies to manage and reduce diabetes in Aboriginal communities and other vulnerable populations.
Disclosures
- Grants/research support, honoraria/consultation fees from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk and Sanofi.
Professor Takashi Kadowaki
 Takashi Kadowaki is Project Professor of Department of Prevention of Diabetes and Life-style Related Diseases, Graduate School of Medicine, the University of Tokyo. He served as Director of the University of Tokyo Hospital. He currently serves as Chairman of the Board of Directors of The Japan Diabetes Society and Chairman of Board of Directors of Japan Society for the Study of Obesity and also serves as Vice President of The Japanese Association of Medical Sciences. His major research interests include molecular basis of insulin resistance and type 2 diabetes and he identified adiponectin receptors, Adipo R1 and Adipo R2 and he has authored more than 600 original articles and reviews. He has received many national and international awards including a Medal with Purple Ribbon from the Japanese Government, Japan Academy Prize, and Manpei Suzuki International Prize for Diabetes Research. He served as an Associate Editor of Diabtetologia, also served as an editorial board member of Diabetes, Diabetes Care and Journal of Clinical Investigation and currently is on the editorial board of Cell Metabolism and Molecular Metabolism and Associate Editor of The Journal of Endocrine Society.
Takashi Kadowaki is Project Professor of Department of Prevention of Diabetes and Life-style Related Diseases, Graduate School of Medicine, the University of Tokyo. He served as Director of the University of Tokyo Hospital. He currently serves as Chairman of the Board of Directors of The Japan Diabetes Society and Chairman of Board of Directors of Japan Society for the Study of Obesity and also serves as Vice President of The Japanese Association of Medical Sciences. His major research interests include molecular basis of insulin resistance and type 2 diabetes and he identified adiponectin receptors, Adipo R1 and Adipo R2 and he has authored more than 600 original articles and reviews. He has received many national and international awards including a Medal with Purple Ribbon from the Japanese Government, Japan Academy Prize, and Manpei Suzuki International Prize for Diabetes Research. He served as an Associate Editor of Diabtetologia, also served as an editorial board member of Diabetes, Diabetes Care and Journal of Clinical Investigation and currently is on the editorial board of Cell Metabolism and Molecular Metabolism and Associate Editor of The Journal of Endocrine Society.
Disclosures
- Research support: MSD Corporation, Daiichi Sankyo Co., Ltd., Novo Nordisk Pharma Ltd., Sanofi K.K., Takeda Pharmaceutical Co., Ltd.
- Endowed chair/Community corporative course: Takeda Pharmaceutical Co., Ltd., TERUMO Corporation, MSD Corporation, Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co ., Ltd.
- Speaker fees: MSD Corporation, Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Kowa Pharmaceutical Co., Ltd., Astellas Pharma Inc., Ono Pharmaceutical Co., Ltd., AstraZeneca K.K., Sumitomo Dainippon Pharma Co., Ltd., Sanofi K.K., Eli Lilly Japan K.K., Nippon Boehringer Ingelheim Co ., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Taisho Pharmaceutical Co., Ltd.
Professor Rosângela Réa
 Rosangela Rea is Professor and Coordinator of the Diabetes Division at the Federal University of Parana in Brazil.
Rosangela Rea is Professor and Coordinator of the Diabetes Division at the Federal University of Parana in Brazil.
Dr Rea has served as primary investigator on more than 30 clinical trials, including cardiovascular outcomes trials, and trials of novel glucagon-like peptide-1 analogues. She is also involved in clinical research, mainly focusing on gestational diabetes.
Dr Rea has authored and co-authored many publications, and was previously Vice President of the Brazilian Diabetes Society from 2000 to 2001 and 2018 to 2019.
Disclosures
- Advisory boards: AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Sanofi
- Speaker’s bureau: AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Sanofi, Takeda
Case 1: Patient Preference and Adherence
After taking part in this case study module you will be able to:
- Explain the recommended steps that should be taken when deciding on a management plan for your patients with diabetes
- Utilize trial data in treatment decisions in order to meet your patients’ preference(s) and optimize treatment adherence
Case 2: Established Cardiovascular Disease and Diabetes
After taking part in this module, you will be able to:
- Explain the importance of cardiovascular risk/disease when choosing the most appropriate glucose-lowering therapies
- Discuss the specific benefits of GLP-1RAs in patients with established cardiovascular disease
Case 3: Weight Management
After taking part in this module, you will be able to:
- Explain the impact of weight when choosing the most appropriate glucose-lowering therapies
- Discuss the specific benefits of GLP-1RAs for weight management
Case 4: GLP-1RAs in patients with renal impairment
After taking part in this module, you will be able to:
- Explain the importance of renal disease when choosing the most appropriate glucose-lowering therapies
- Discuss the specific benefits of GLP-1RAs in patients with renal impairment

CME credits are no longer available.
Date of expiration: 5th February 2020
In support of improving patient care, this activity has been planned and implemented by North American Center for Continuing Medical Education (NACCME), Imedex, and Springer Healthcare IME. NACCME is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physicians
NACCME designates AMA PRA Category 1 Credit™ for the following activities:
- Completion of each case study: 0.25 AMA PRA Category 1 Credit™
- Completion of all case studies: 1 AMA PRA Category 1 Credit™
How To Claim Your Credits
To claim your credits for participating in the accredited activities, you will need to complete the activity then fill out the short evaluation form at the end of each module and tick the appropriate box.
Disclosure of Relevant Financial Relationships
NACCME, LLC is an independent provider(s) of continuing medical education. NACCME, LLC has no proprietary or financial interest in medical or healthcare products over which the FDA (USA) or EMA (EU) has regulatory authority.
NACCME, LLC assures that all educational materials are reviewed to ensure for the absence of commercial bias, fair balance, scientific objectivity, and levels of evidence. The educational activity will not be influenced by commercial organizations and will not conflict with any other scheduled educational activities.
NACCME requires instructors, planners, managers, and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COIs are thoroughly vetted and resolved according to PIM policy. The existence or absence of COI for everyone in a position to control content will be disclosed to participants prior to the start of each activity.
Planning Committee
In addition to the speaker faculty, NACCME and Springer Healthcare IME planners and staff include Chris Bolwell, Jennifer Ilcyn, Marie Le Solliec, and Rebecca Cox.
Chris Bolwell is a shareholder of GlaxoSmithKline. GlaxoSmithKline is not supporting this activity, nor does any content relate to their product/service.
Jennifer Ilcyn, Marie Le Solliec, and Rebecca Cox have no financial relationships to disclose.
The information and data provided in this program was updated and correct at the time of the program development, but may be subject to change.
